Galectin-9 inhibition of the MIF-CD74/CD44 pathway suppresses chronic arthritis
Meiling Li1,2,9 ∙ Min-Kyung Nam1,9 ∙ Jung Gon Kim3,4,9 ∙ Juyeon Kang3 ∙ Se-Hyeon Park1,3 ∙ Su-Hyun Lee1,3 ∙ Chaerin Kim1,3 ∙ David Song5 ∙ Jingchun Jin6∙ Seung-Ah Yoo1,3 ∙ Richard Bucala7 ∙ Wan-Uk Kim1,3,8
1) Department of Biomedicine & Health Sciences, Department of Medical Life Sciences, College of Medicine, The Catholic University of Korea, Seoul, Korea
2) Department of Rheumatology and Immunology, The Second Affiliated Hospital of Soochow University, Suzhou, China
3) Center for Integrative Rheumatoid Transcriptomics and Dynamics, The Catholic University of Korea, Seoul, Korea
4) Department of Internal Medicine, Inje University Ilsan Paik Hospital, Goyang, Korea
5) GBIOLOGICS, Seongnam, Korea
6) Department of Immunology of Yanbian University Hospital, Yanji, Jilin Province, China
7) Department of Internal Medicine, Yale University School of Medicine, New Haven, CT, USA
8) Department of Internal Medicine, The Catholic University of Korea, Seoul, Korea
9) These authors contributed equally
Abstract
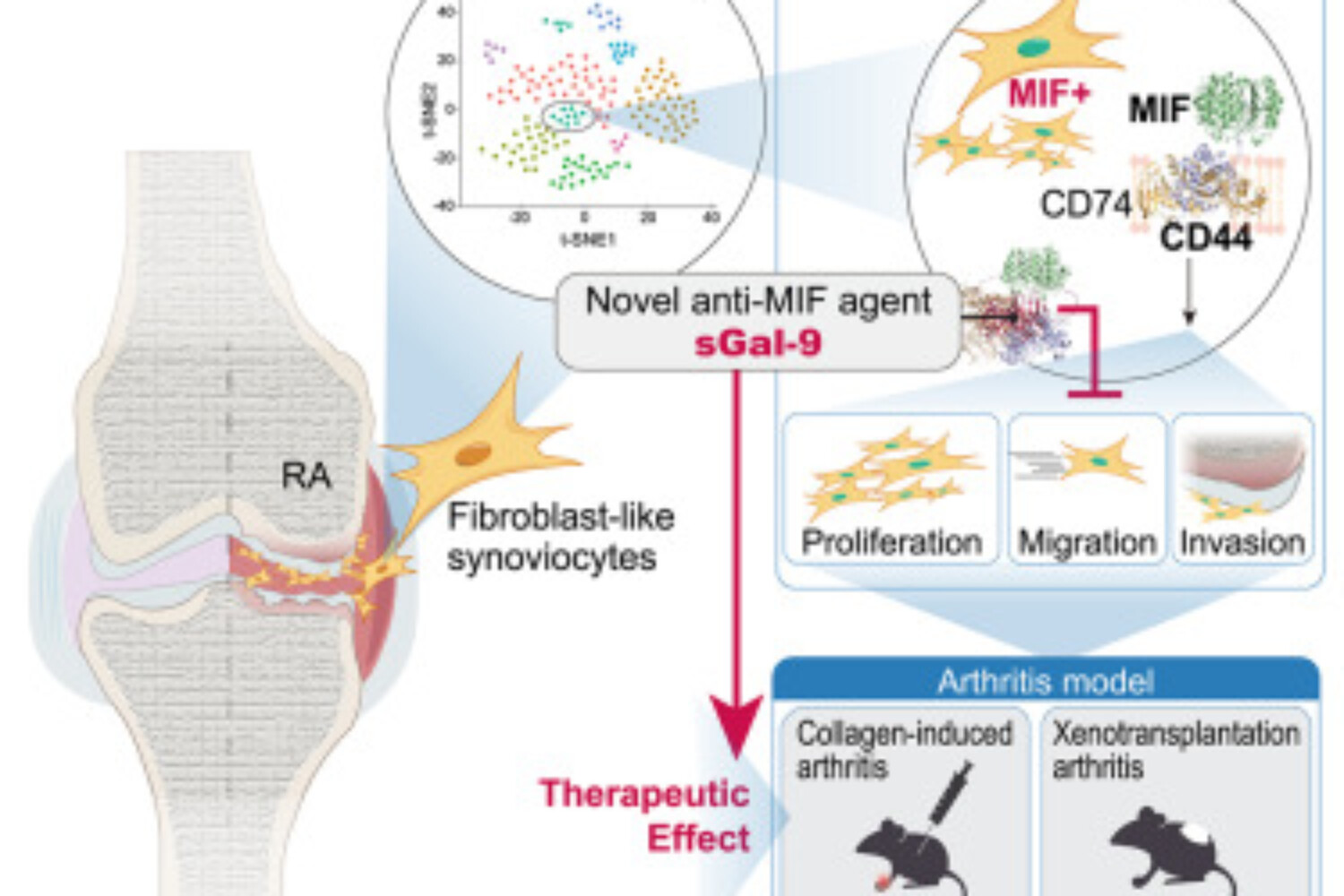
The destructive potential of rheumatoid arthritis (RA) lies in the aggressive behavior of fibroblast-like synoviocytes (FLSs), which actively contribute to the erosion of cartilage and bone and may persist even in the face of apparent clinical remission. Therapeutic approaches targeting RA-FLSs have been developed to treat RA; however, there are no clinically approved drugs available at present. Here, single-cell RNA sequencing of RA-FLSs identified a distinct macrophage migration inhibitory factor (MIF)high subset with mitochondrial and endoplasmic reticulum dysfunction. MIFhigh conditions led to increased survival, proliferation, and migration of FLSs, along with the upregulation of CD44 and the CD44v6 isoform expression. We next explored whether a stable, recombinant form of galectin-9 (sGal-9), which acts as a CD44 blockade, regulates the MIF-induced aggressive phenotype of RA-FLSs. We found that sGal-9 remarkably reduced the increased proliferation, migration, and invasion of RA-FLSs by inhibiting the MIF-CD44 pathway. Moreover, both local and systemic administration of sGal-9 substantially inhibited excessive cartilage and bone destruction by RA-FLSs in a xenotransplantation arthritis model and alleviated the severity of collagen-induced arthritis in mice, comparable to Enbrel and tofacitinib. Conclusively, these data suggest that sGal-9 is effective at repressing destructive phenotypes of RA-FLSs as a novel anti-MIF agent.